C the magnetic quantum number ml. Chemistry questions and answers.
The Orientation In Space Of An Atomic Orbital Is Associated With What Socratic
Thus s orbital corresponds to spherical shape with the atomic nucleus at its centre.

. The orientation in space of an atomic orbital is associated with. The angular momentum quantum number l. Describe regions of space in which one is most likely to find an electron.
The orientation in space of an atomic orbital is associated with A the principal quantum number n. 1 5 4 3 2. Answer to Solved The orientation in space of an atomic orbital is.
The orientation in space of an atomic orbital is associated with the magnetic quantum number m. Sub-shells represent the lines obtained in atomic spectrums. A v 1t.
The magnetic quantum number ml Atomic orbitals developed using quantum mechanics. The properties of the atomic orbital are actually dependent on the quantum numbers. The size of an atomic orbital is associated with.
The energy of an electron in the hydrogen atom is determined by. The Magnetic Quantum Number mL D. The spin quantum number ms.
The Principal Quantum Number n B. For example given the so-called 2p subshell we have that l 1 for all 2l1 three 2p atomic. Describe regions of space in which one is most likely to find an electron.
View the full answer. The Orientation in space of an atomic orbital is associated with Share Question Share to WhatsApp Question 17 Chemistry Quantum Numbers. Generally an atom consists of electrons that are fixed inside the electronic orbitals.
The period t is the time it takes one complete wavelength to pass a point in space. B the principle quantum number n C the spin quantum number ms D the angular momentum quantum number l Expert Answer. It is denoted by m.
S orbital. The Angular Momentum Quantum Number l C. The principal quantum number n B.
Atomic Orbitals and Quantum Numbers. In simpler terms atomic orbital can be described as the physical bounded region or space where the electrons are present. Chemistry questions and answers.
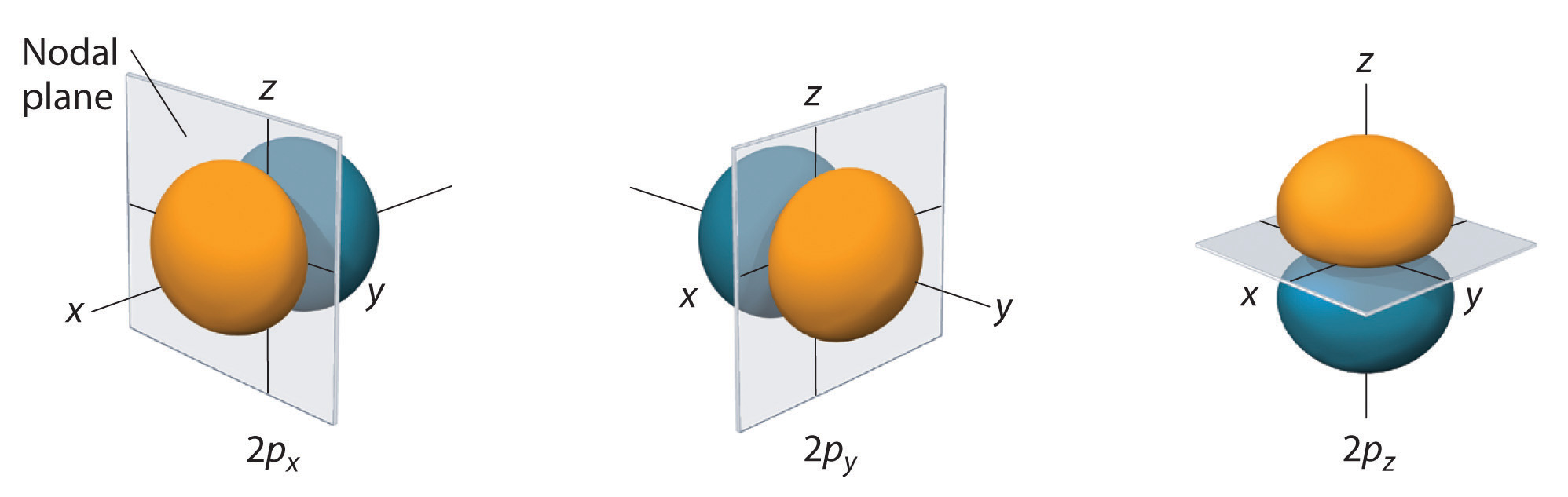
S and p atomic orbital shapes Magnetic Quantum Number m Gives the orientation of the orbital in space. In atomic theory and quantum mechanics an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. S orbitals are present in all principal energy levels.
The principal quantum number n. In other words the value of m describes whether an orbital lies along the x- y- or z-axis on a three-dimensional graph with the nucleus of the atom at the origin. Though they do exist at specific energy levels as in the Bohr model their wave property makes it impossible to predict exactly where an electron is at a given moment and so we can only speak of the probability of finding the electron at any given location.
N 3. Atomic configuration Heisenberg configuration. For s orbital Azimuthal quantum number 0 and the magnetic quantum number m 0 hence s orbitals have unique orientation in space.
A the magnetic and spin quantum numbers together. The orientation in space of an atomic orbital is associated with the angular momentum quantum number 0 Select one. The principal quantum number n only.
The orbital represents a space where there is a high. Answer to Solved The orientation in space of an atomic orbital is. The principal quantum number n.
Its associated with its magnetic quantum number m_l. This preview shows page 13 - 16 out of 16 pagespreview shows page 13 - 16 out of 16 pages. Describe regions of space in which one is most likely to find an electron.
The Orientation in space of an atomic orbital is associated with. The angular momentum quantum number l. The orientation in space of an atomic orbital is associated with A the.
D the spin quantum number ms. The orientation in space of an atomic orbital is associated with. B the angular momentum quantum number 1.
For every value of n there is one s orbital ie. The spin quantum numberms. If we look at any atomic orbital it is generally associated with three quantum numbers.
The angular momentum quantum number I. M can take on any value from -l to l. It gives information about the orientation of the different orbitals that are present in the sub-shells.
The size of the orbital is governed and decided by the principal quantum number n which is dependent on the overall average distance between the number of electrons as well as the nucleus. This problem has been solved. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleusThe term atomic orbital may also refer to the physical region or space where.
In the periodic table it gives information about groups. The magnetic quantum number is concerned with the orbitals orientation in space. The size of an atomic orbital is associated with A the principal quantum number n.
Based on this information what is the mathematical relationship between v and t. Size of atomic orbital. Governed by the angular momentum quantum number l orientation in space.
The shape of an atomic orbital is associated with. The orientation in space of an atomic orbital is associated with. Option C is the correct answer.
The shape of an atomic orbital is associated with A. Governed by the principal quantum number n shape of atomic orbital. A given set of atomic orbitals specified by the angular momentum quantum number l has 2l 1 degenerate orbitals of equal energy each of which corresponds to a unique m_l in the set -l -l1.
Governed by the magnetic quantum number ml. Unlike the Bohr model electrons are not confined to specific circular orbit. True False Previous page Next page Return to.
The shape of an atomic orbital is associated with. E None of these choices is correct. How many electrons are in the 4d orbitals of Tc.
Which of the following is a correct set of quantum numbers for an electron in a 3d orbital. See the answer See the answer done loading. The orbitals shape is explained by the angular quantum number.
The orientation in space of an atomic orbital is associated with. They were discovered from fine lines present on the atomic spectrum.

Solved The Orientation In Space Of An Atomic Orbital Is Chegg Com

Chemistry Atomic Orbital Shapes Ggca Science Lab Science Lab Shapes Atomic Structure

Solved The Orientation In Space Of An Atomic Orbital Is Chegg Com
0 Comments